現階段腐蝕實驗是探究金屬腐蝕(shi)以及防護的主要手段,通過腐蝕實驗可以探究金屬發生腐蝕的規律及機理、檢查并篩選金屬材料的材質、估算金屬材料的使用壽命、分析金屬材料腐蝕事故的原因以及驗證防腐蝕的效果等。目前探究金屬腐蝕以及防護的方式有多種,如表面分析法、失重法以及電化學法。因為大部分金屬產生的腐蝕都是電化學腐蝕,而腐蝕過程中發生的電化學反應為電化學腐蝕的本質,所以金屬/電解質界面(雙電層)的電化學性質被大量使用于探究金屬腐蝕發生的規律、腐蝕產生的機理等方面。因此,在現有的研究金屬腐蝕與防護的方法中,電化學方法是一種較為重要的方法。腐蝕電化學法能夠按照腐蝕金屬電極特點的不同而分為以下三種類型:①. 電化學動力研究方法,指利用控制極化電流及電極電位來測定腐蝕體系中的熱力學參數;②. 獨用的腐蝕電化學測量跟研究法,指按照金屬電化學腐蝕的獨特性建立相應的電化學測量跟研究方法;③. 通過模擬裝置來探究具有獨特腐蝕形態的電化學測試技術,如模擬SSRT裂紋尖端的裝置、研究縫隙腐蝕的閉塞電池等。這幾種方法中最基礎的為電化學動力法。
電(dian)(dian)極(ji)電(dian)(dian)位以及(ji)電(dian)(dian)流密度為(wei)腐蝕電(dian)(dian)化(hua)學(xue)(xue)實驗所要(yao)獲得的(de)重要(yao)參數,其(qi)中電(dian)(dian)極(ji)電(dian)(dian)位表(biao)示電(dian)(dian)解(jie)液-金屬(shu)界面(mian)的(de)特性和(he)結(jie)構;電(dian)(dian)流密度表(biao)示金屬(shu)材料表(biao)面(mian)上單位面(mian)積(ji)內電(dian)(dian)化(hua)學(xue)(xue)反應(ying)進行的(de)快慢(man)。絕大多(duo)數電(dian)(dian)化(hua)學(xue)(xue)測(ce)(ce)試都是測(ce)(ce)定電(dian)(dian)極(ji)電(dian)(dian)位跟電(dian)(dian)流密度這兩者間的(de)關聯。
與其他電化學過程(cheng)(cheng)(如電鍍、電解及化學電源等)相比,金屬電化學腐蝕測(ce)量過程(cheng)(cheng)的對象是金屬電極,該過程(cheng)(cheng)有如下特點:
1. 金屬(shu)發(fa)生腐蝕的(de)整(zheng)個腐蝕體系由數個電(dian)極(ji)反(fan)應耦合(he)而成,同(tong)時在整(zheng)個電(dian)極(ji)表面上也(ye)發(fa)生著數個電(dian)極(ji)反(fan)應,所以與只具有(you)一個電(dian)極(ji)反(fan)應的(de)電(dian)極(ji)系統相(xiang)比,其在分析和(he)處理(li)腐蝕電(dian)化學實驗結果(guo)上有(you)著一定特別之處。
2. 電(dian)(dian)(dian)極金屬材料發生(sheng)陽極溶解反(fan)應(ying),即腐蝕金屬自(zi)身參(can)與的(de)反(fan)應(ying)是(shi)電(dian)(dian)(dian)極系統中電(dian)(dian)(dian)極反(fan)應(ying)中的(de)一種(zhong)。
3. 測量過程中(zhong)不可(ke)以(yi)(yi)只探究(jiu)整個電極(ji)表面(mian)總的電化學行為,因(yin)為電極(ji)表面(mian)表現為多層結構,金屬(shu)電極(ji)上有(you)著腐蝕(shi)產物(wu)銹層、腐蝕(shi)孔(kong)及表面(mian)膜(mo),導致電極(ji)表面(mian)具有(you)不光滑(hua)的特(te)點,容易發(fa)生各種形(xing)式的局部腐蝕(shi),所以(yi)(yi)有(you)必要發(fa)展(zhan)如微區(qu)電化學測試之(zhi)類的能夠表征電極(ji)表面(mian)不均(jun)勻性的研(yan)究(jiu)方法。
4. 腐蝕金屬的電極反應相對于(yu)其他一些(xie)電化學過程而言比較(jiao)緩慢(man)。
此外(wai),腐蝕電(dian)化學(xue)測試方(fang)(fang)法為原位(wei)技術,能夠(gou)比較真實地反應金屬電(dian)極(ji)表面發生的實際腐蝕,擁有(you)較強的靈(ling)敏度、操作簡單容易實施且實時性(xing)好的優點。電(dian)化學(xue)實驗常(chang)用的方(fang)(fang)法有(you)極(ji)化曲線、交流阻抗及電(dian)位(wei)掃描等。
極(ji)(ji)(ji)化(hua)曲線的(de)(de)(de)測量有(you)利(li)于(yu)研究電(dian)(dian)(dian)(dian)(dian)極(ji)(ji)(ji)過(guo)程的(de)(de)(de)影響因(yin)素和機(ji)理。眾所(suo)周知,當我們探究可(ke)(ke)逆(ni)電(dian)(dian)(dian)(dian)(dian)池的(de)(de)(de)反(fan)(fan)應(ying)時電(dian)(dian)(dian)(dian)(dian)極(ji)(ji)(ji)上基本(ben)上是(shi)不(bu)存在電(dian)(dian)(dian)(dian)(dian)流的(de)(de)(de),各(ge)個電(dian)(dian)(dian)(dian)(dian)極(ji)(ji)(ji)的(de)(de)(de)反(fan)(fan)應(ying)基本(ben)都在平(ping)衡(heng)(heng)狀(zhuang)態下發生(sheng),所(suo)以該(gai)反(fan)(fan)應(ying)為可(ke)(ke)逆(ni)的(de)(de)(de)。但是(shi)一旦存在電(dian)(dian)(dian)(dian)(dian)流通(tong)過(guo),電(dian)(dian)(dian)(dian)(dian)極(ji)(ji)(ji)原(yuan)本(ben)的(de)(de)(de)平(ping)衡(heng)(heng)狀(zhuang)態就被打(da)破,進而(er)(er)導(dao)(dao)致電(dian)(dian)(dian)(dian)(dian)極(ji)(ji)(ji)電(dian)(dian)(dian)(dian)(dian)位偏離(li)原(yuan)本(ben)的(de)(de)(de)平(ping)衡(heng)(heng)電(dian)(dian)(dian)(dian)(dian)位值,導(dao)(dao)致電(dian)(dian)(dian)(dian)(dian)極(ji)(ji)(ji)反(fan)(fan)應(ying)處于(yu)一種(zhong)不(bu)可(ke)(ke)逆(ni)的(de)(de)(de)狀(zhuang)態,不(bu)可(ke)(ke)逆(ni)程度隨著電(dian)(dian)(dian)(dian)(dian)極(ji)(ji)(ji)電(dian)(dian)(dian)(dian)(dian)流密(mi)度的(de)(de)(de)升高(gao)而(er)(er)增(zeng)強,即所(suo)謂的(de)(de)(de)電(dian)(dian)(dian)(dian)(dian)極(ji)(ji)(ji)極(ji)(ji)(ji)化(hua)就是(shi)指由于(yu)電(dian)(dian)(dian)(dian)(dian)流通(tong)過(guo)電(dian)(dian)(dian)(dian)(dian)極(ji)(ji)(ji)而(er)(er)導(dao)(dao)致電(dian)(dian)(dian)(dian)(dian)位偏離(li)平(ping)衡(heng)(heng)值的(de)(de)(de)一種(zhong)現(xian)狀(zhuang),極(ji)(ji)(ji)化(hua)曲線即表示電(dian)(dian)(dian)(dian)(dian)極(ji)(ji)(ji)電(dian)(dian)(dian)(dian)(dian)位與電(dian)(dian)(dian)(dian)(dian)流密(mi)度兩者間(jian)的(de)(de)(de)關(guan)系,其(qi)測試有(you)以下幾種(zhong)方法。
a. 恒電位法
恒電(dian)(dian)(dian)(dian)位(wei)(wei)(wei)法(fa)即將被研究(jiu)的(de)(de)(de)電(dian)(dian)(dian)(dian)極(ji)電(dian)(dian)(dian)(dian)位(wei)(wei)(wei)固定(ding)在(zai)不(bu)同的(de)(de)(de)電(dian)(dian)(dian)(dian)位(wei)(wei)(wei)上,然(ran)后測試(shi)對應(ying)(ying)電(dian)(dian)(dian)(dian)位(wei)(wei)(wei)下的(de)(de)(de)電(dian)(dian)(dian)(dian)極(ji)電(dian)(dian)(dian)(dian)流(liu)密度(du)(du),在(zai)實際(ji)應(ying)(ying)用(yong)過程(cheng)中(zhong)使(shi)用(yong)較為(wei)普(pu)遍的(de)(de)(de)是(shi)靜(jing)態(tai)法(fa)及動(dong)態(tai)法(fa)。所謂(wei)靜(jing)態(tai)法(fa)是(shi)指控制(zhi)(zhi)電(dian)(dian)(dian)(dian)極(ji)電(dian)(dian)(dian)(dian)位(wei)(wei)(wei)為(wei)某(mou)一(yi)個(ge)特定(ding)值(zhi)(zhi),測量(liang)(liang)相(xiang)對應(ying)(ying)電(dian)(dian)(dian)(dian)位(wei)(wei)(wei)下的(de)(de)(de)電(dian)(dian)(dian)(dian)流(liu)密度(du)(du),且依次(ci)測定(ding)整個(ge)電(dian)(dian)(dian)(dian)極(ji)電(dian)(dian)(dian)(dian)位(wei)(wei)(wei)下的(de)(de)(de)電(dian)(dian)(dian)(dian)流(liu)密度(du)(du),從而得(de)到整個(ge)極(ji)化(hua)(hua)曲線(xian);其(qi)(qi)次(ci)動(dong)態(tai)法(fa)指控制(zhi)(zhi)電(dian)(dian)(dian)(dian)極(ji)電(dian)(dian)(dian)(dian)位(wei)(wei)(wei)按照較為(wei)緩慢的(de)(de)(de)速度(du)(du)不(bu)停(ting)地變化(hua)(hua),并且測量(liang)(liang)相(xiang)對應(ying)(ying)電(dian)(dian)(dian)(dian)位(wei)(wei)(wei)下的(de)(de)(de)電(dian)(dian)(dian)(dian)流(liu)值(zhi)(zhi),瞬(shun)時電(dian)(dian)(dian)(dian)流(liu)與其(qi)(qi)相(xiang)對應(ying)(ying)的(de)(de)(de)電(dian)(dian)(dian)(dian)位(wei)(wei)(wei)關系曲線(xian)即為(wei)極(ji)化(hua)(hua)曲線(xian)。這兩種方法(fa)中(zhong)較為(wei)廣泛使(shi)用(yong)的(de)(de)(de)是(shi)動(dong)態(tai)法(fa)測定(ding)極(ji)化(hua)(hua)曲線(xian),該(gai)方法(fa)的(de)(de)(de)優(you)點(dian)在(zai)于掃描速度(du)(du)可(ke)以控制(zhi)(zhi)、可(ke)以自動(dong)測量(liang)(liang)并繪制(zhi)(zhi)極(ji)化(hua)(hua)曲線(xian),其(qi)(qi)測量(liang)(liang)的(de)(de)(de)結(jie)果有較高(gao)的(de)(de)(de)重現(xian)性,對于那(nei)些需要比較的(de)(de)(de)實驗該(gai)方法(fa)為(wei)首選。
b. 恒電流法
恒電(dian)(dian)(dian)流(liu)法是指固(gu)定電(dian)(dian)(dian)極體系(xi)的電(dian)(dian)(dian)流(liu)密度為某一(yi)特定值,測定跟電(dian)(dian)(dian)流(liu)密度相對(dui)應的電(dian)(dian)(dian)極電(dian)(dian)(dian)位。恒電(dian)(dian)(dian)流(liu)法測量極化(hua)曲(qu)線在測定過程(cheng)中電(dian)(dian)(dian)極很難達到一(yi)個穩定的狀態,所以在實際測量過程(cheng)中一(yi)般當電(dian)(dian)(dian)位接近(jin)穩定的時候(hou)即可以讀值。
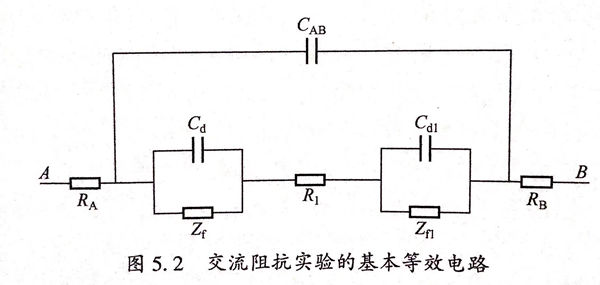
典型的動電位極化曲線如圖5.1所示。圖中Eb為金屬材料的點蝕電位,Ep為保護電位。同樣的實驗狀態下點蝕電位(Eb)值越大則意味著金屬產生點腐蝕的傾向越低;當幾種金屬材料的點蝕電位值相當,只有將點蝕電位和保護電位綜合考慮才能評價金屬的耐蝕能力,(Eb-Ep)差值越低表明材料鈍化膜修復能力越強,耐孔蝕性能越優,因而保護電位(Ep)和點蝕電位(Eb)是被用來表示金屬耐孔腐蝕能力大小的基本參數。在E>Eb的條件下,點蝕必然會發生,不但原來具有的蝕孔會長大而且還會產生新的蝕孔;在E<Ep的情況下不會發生點蝕,原來的孔蝕不會長大而且新的蝕孔也不會產生;在Ep<E<Eb條件下,孔蝕存在,原有的蝕孔會接著擴展并生長,但是新蝕孔不會產生。
電化學阻抗譜(Electrochemical Impedance Spectroscopy,EIS),在早期的電化學文獻中電化學阻抗又被稱為交流阻抗(Alternating Current impedance,AC im-pedance).電化學阻抗原先被用于電學中來探究線性電路網絡頻率響應特征,后來被用在電極上,進而成為電化學的研究方式。電化學阻抗譜的原理是指向電化學體系施予一頻率各異的小振幅交流電動勢,測定正弦波頻率(ω)的改變對該電動勢與電流信號比值產生的影響,即測定阻抗隨著正弦波頻率(ω)的變化,也可以通過測定阻抗的相位角Φ隨ω的變化來分析電極材料、腐蝕機理、導電材料、電極過程的動力學等方面的機理。采用小振幅的電信號既能夠防止給系統帶來較大的影響,同時又能夠讓擾動跟響應體系之間表現為近似線性的關系,進而讓測量的結果數學處理更容易。此外,電化學阻抗譜是通過測量過程中獲得的頻率比較寬的阻抗譜探究電極的,所以相對于另外一些電化學法其能夠得到電極界面結構和動力學信息。例如:通過阻抗譜形狀能夠探究金屬電極發生腐蝕的機理;探究金屬表面上保護膜的阻抗特征;對腐蝕金屬進行電化學阻抗測量可以獲得極化電阻(Rp);對腐蝕的金屬材料進行電化學阻抗譜測量,能夠了解動力學參數進而來研究金屬材料抗腐蝕能力的強弱等。因此,電化學阻抗譜成為近年來探究金屬發生腐蝕與采取相應防護措施的重要方式。
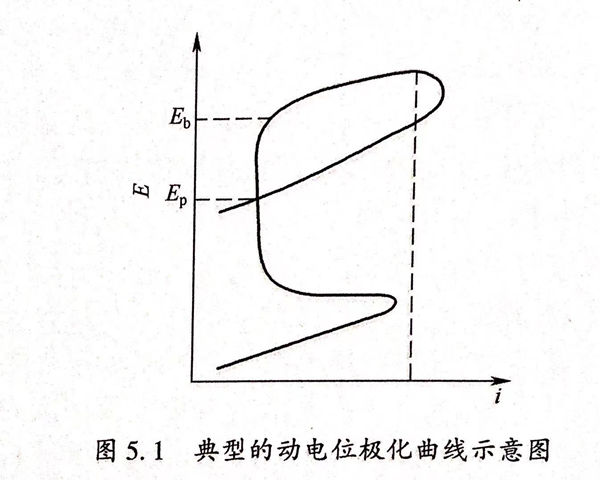
電(dian)(dian)(dian)(dian)化(hua)學(xue)(xue)阻抗(kang)(EIS)測試把電(dian)(dian)(dian)(dian)化(hua)學(xue)(xue)系(xi)統(tong)(tong)作為一個等(deng)效(xiao)電(dian)(dian)(dian)(dian)路,交流阻抗(kang)實驗(yan)的(de)(de)(de)(de)基本等(deng)效(xiao)電(dian)(dian)(dian)(dian)路如圖5.2所示(shi)。該(gai)電(dian)(dian)(dian)(dian)路的(de)(de)(de)(de)組成(cheng)(cheng)元(yuan)件(jian)有電(dian)(dian)(dian)(dian)阻(R:金屬材料對(dui)電(dian)(dian)(dian)(dian)流的(de)(de)(de)(de)阻攔功能)、電(dian)(dian)(dian)(dian)感(L:于電(dian)(dian)(dian)(dian)路中對(dui)交流電(dian)(dian)(dian)(dian)的(de)(de)(de)(de)阻礙功能)及(ji)電(dian)(dian)(dian)(dian)容(C:電(dian)(dian)(dian)(dian)路中對(dui)交流電(dian)(dian)(dian)(dian)所引起的(de)(de)(de)(de)阻礙作用)等(deng),這些元(yuan)件(jian)按照串聯或者并聯的(de)(de)(de)(de)方式組合(he)起來形成(cheng)(cheng)一個等(deng)效(xiao)電(dian)(dian)(dian)(dian)路。測量電(dian)(dian)(dian)(dian)化(hua)學(xue)(xue)阻抗(kang)能夠(gou)確定(ding)等(deng)效(xiao)電(dian)(dian)(dian)(dian)路的(de)(de)(de)(de)組成(cheng)(cheng)方式及(ji)各(ge)組成(cheng)(cheng)元(yuan)件(jian)的(de)(de)(de)(de)值、通過(guo)這些元(yuan)件(jian)的(de)(de)(de)(de)電(dian)(dian)(dian)(dian)化(hua)學(xue)(xue)含義就可以分析(xi)電(dian)(dian)(dian)(dian)化(hua)學(xue)(xue)電(dian)(dian)(dian)(dian)極過(guo)程的(de)(de)(de)(de)性(xing)質和電(dian)(dian)(dian)(dian)化(hua)學(xue)(xue)系(xi)統(tong)(tong)的(de)(de)(de)(de)結構。